LEC 5 Introduction
In this section we are going to study further division of atom, inside view of atom, building block of atoms, difference between two different atoms etc.
before discovery of subatomic particles scientist were think that the continued subdivisions of matter would ultimately yield atoms which would not be further divisible. The word ‘atom’ has been derived from the Greek word ‘a-tomio’ which means ‘uncutable’ or ‘non-divisible’ but after discovery of neutron, protons and electrons it is prove that atom is divided into these subatomic particles and even A proton can be divided into 2 Ups and a Down quark, and a neutron into 2 Downs and an Up quark. In general the quark is the elementary particle from which protons and neutron are formed.
The atomic theory of matter was first proposed on a firm scientific basis by John Dalton, a British school teacher in 1808. His theory, called Dalton’s atomic theory, regarded the atom as the ultimate particle of matter.
The major problems before the scientists at that time were:
1) to account for the stability of atom after the discovery of sub-atomic particles,
2) to compare the behaviour of one element from other in terms of both physical and chemical properties,
3) to explain the formation of different kinds of molecules by the combination of different atoms and,
4) to understand the origin and nature of the characteristics of electromagnetic radiation absorbed or emitted by atoms
Discovery of Electron
In mid 1850s many scientists mainly Faraday, Davy, Crookes and J.J. Thomson began to study electrical discharge in partially evacuated tubes, known as cathode ray discharge tubes.
A cathode ray tube is made of glass containing two thin pieces of metal, called electrodes, sealed in it. The electrical discharge through the gases could be observed only at very low pressures(0.01 – 0.03 mm) and at very high voltages(10,000 volts). The pressure of different gases could be adjusted by evacuation. When sufficiently high voltage is applied across the electrodes, current starts flowing through a stream of particles moving in the tube from the negative electrode (cathode) to the positive electrode (anode). These were called cathode rays or cathode ray particles.
The flow of current from cathode to anode was further checked by making a hole in the anode and coating the tube behind anode with phosphorescent material zinc sulphide. When these rays, after passing through anode, strike the zinc sulphide coating, a bright spot on the coating is developed(same thing happens in a television set).
The results of these experiments are summarised below:
(i) The cathode rays start from cathode and move towards the anode.
(ii) These rays themselves are not visible but their behaviour can be observed with the help of certain kind of materials (fluorescent or phosphorescent) which glow when hit by them. Television picture tubes are cathode ray tubes and television pictures result due to fluorescence on the television screen coated with certain fluorescent or phosphorescent materials.
(iii) In the absence of electrical or magnetic field, these rays travel in straight lines.
(iv) In the presence of electrical or magnetic field, the behaviour of cathode rays are similar to that expected from negatively charged particles, suggesting that the cathode rays consist of negatively charged particles, called electrons.
(v) The characteristics of cathode rays (electrons) do not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube.
(vi) They rotate a light wheel placed in their paths. This shows that cathode rays contain material particles having both mass and velocity.
(vii) The mass of a particle present in cathode rays is found to be 1/1837 of H-atom. This showsthat the particle is of sub-atomic nature.
Thus, we can conclude that electrons are basic constituent of all the atoms having negative charged (in 1897 by J.J. Thomson).
Charge to Mass Ratio of Electron
In 1897, British physicist J.J. Thomson discover the electrons and measured the ratio of electrical charge (e) to the mass of electron (me ) by using cathode ray tube and applying electrical and magnetic field perpendicular to each other as well as to the path of electrons (Fig. 2.2). Thomson argued that the amount of deviation of the particles from their path in the presence of electrical or magnetic field depends upon:
(i) the magnitude of the negative charge on the particle, greater the magnitude of the charge on the particle, greater is the interaction with the electric or magnetic field and thus greater is the deflection.
ii) the mass of the particle — lighter the particle, greater the deflection.
(iii) the strength of the electrical or magnetic field — the deflection of electrons from its original path increases with the increase in the voltage across the electrodes, or the strength of the magnetic field.
By carrying out accurate measurements on the amount of deflections observed by the electrons on the electric field strength or magnetic field strength, Thomson was able to determine the value of e/me as:
Where me is the mass of the electron in kg and e is the magnitude of the charge on the electron in coulomb (C). Since electrons are negatively charged, the charge on electron is –e.
Charge on the Electron
R.A. Millikan devised a method known as oil drop experiment (1906-14), to determine the charge on the electrons. He found that the charge on the electron to be – 1.6 × 10-19 C. The present accepted value of electrical charge is – 1.6022 × 10-19 C. The mass of the electron (me) was determined by combining these results with Thomson’s value of e/me ratio.
Mass of electrone = 9.1094×10-31 kg
Discovery of Proton
In 1886, Eugen Goldstein discovered canal rays (also known as anode rays) and showed that they were positively charged particles (ions) produced from gases. However, since particles from different gases had different values of charge-to-mass ratio (e/m), they could not be identified with a single particle, unlike the negative electrons discovered by J. J. Thomson.
Following the discovery of the atomic nucleus by Ernest Rutherford in 1911, In 1917, (in experiments reported in 1919) Rutherford proved that the hydrogen nucleus is present in other nuclei, a result usually described as the discovery of the proton.
Rutherford bombarded gases with alpha particles and in case of Nitrogen it is observed that that the alpha particle actually entered the nitrogen nucleus which immediately ejected a proton.
The characteristics of these positively charged particles are listed below:
(i) unlike cathode rays, the positively charged particles depend upon the nature of gas present in the cathode ray tube. These are simply the positively charged gaseous ions.
(ii) The charge to mass ratio of the particles is found to depend on the gas from which these originate.
(iii) Some of the positively charged particles carry a multiple of the fundamental unit of electrical charge.
(iv) The behavior of these particles in the magnetic or electrical field is opposite to that observed for electron or cathode rays.
The smallest and lightest positive ion was obtained from hydrogen and was called proton was characterised in 1919.
Discovery of Neutron
Because of two facts that is mass of atom is not eaqual to mass of all protons and second is proton proton repulsion due to same charge, needs further investigation on the atomic subparticles.
Ernest Rutherford in 1921 postulated a particle called the “neutron,” having a similar mass as a proton but electrically neutral. Rutherford imagined a paired proton and electron somehow joined in one particle. One major problem with Rutherford’s “neutron theory”—not much evidence.
In 1932, James Chadwick was working at the same lab that lab was directed by Rutherford.he prove that the capture of the a-particle by the Be-9 nucleus may be supposed to result in the formation of a C-12 nucleus and the emission of the neutron of mass 1 and charge 0.
Dalton’s indivisible atom is composed of sub-atomic particles carrying positive, negative and neutral charges protons, electrons and neutrons. there physical properties are given as:
ATOMIC MODELS
Different atomic models were proposed to explain the distributions of charged particles in an atom like the cubic model (1902), the plum-pudding model (1904), the Saturnian model(1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). Although some of these models were not able to explain the stability of atoms, two of these models, proposed by J. J. Thomson and Ernest Rutherford are discussed below.
Thomson Model of Atom
J. J. Thomson, in 1898, proposed this model and many different names are given to this model, for example, plum pudding, raisin pudding or watermelon. This model can be visualised as a pudding or watermelon of positive charge with plums or seeds (electrons) embedded into it. An important feature of this model is that 1) an atom possesses a spherical shape (radius approximately 10–10 m) in which the positive charge is uniformly distributed.
2) The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement
3) the mass of the atom is assumed to be uniformly distributed over the atom.
Although this model was able to explain the overall neutrality of the atom, but was not consistent with the results of later experiments. Thomson was awarded Nobel Prize for physics in 1906, for his theoretical and experimental investigations on the conduction of electricity by gases.
Rutherford’s Nuclear Model of Atom
Rutherford overturned Thomson's model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny, heavy nucleus.
Rutherford presented his own physical model for subatomic structure, as an interpretation for the unexpected experimental results. In it, the atom is made up of a central charge (this is the modern atomic nucleus, though Rutherford did not use the term "nucleus" in his paper) surrounded by a cloud of (presumably) orbiting electrons. In this May 1911 paper, Rutherford only commits himself to a small central region of very high positive or negative charge in the atom.
in this experiment a stream of high energy α–particles from a radioactive source was directed at a thin foil (thickness ∼ 100 nm) of gold metal. The thin gold foil had a circular fluorescent zinc sulphide screen around it. Whenever α–particles struck the screen, a tiny flash of light was produced at that point.
He was observed that :
(i) most of the α– particles passed through the gold foil without deflected.
(ii) a few of the α–particles was deflected by small angles.
(iii) a very few of the α– particles (∼1 in 20,000) bounced back, that is, were deflected by nearly 180°.
On the basis of the observations, Rutherford drew the following conclusions regarding the structure of atom :
(i) Most of the space in the atom is empty as most of the α–particles passed through the foil without deflected.
(ii) A few positively charged α– particles were deflected. The deflection must be due to enormous repulsive force showing that the positive charge of the atom is not spread throughout the atom as Thomson had presumed. The positive charge has to be concentrated in a very small volume that repelled and deflected the positively charged α– particles.
(iii) Calculations by Rutherford showed that the volume occupied by the nucleus is negligibly small as compared to the total volume of the atom. The radius of the atom is about 10-10 m while radius or nucleus is about 10-15 m.
On the basis of above observations and conclusions, Rutherford proposed the nuclear model of atom (after the discovery of protons). According to this model :
(i) The positive charge and most of the mass of the atom was densely concentrated in extremely small region. This very small portion of the atom was called nucleus by Rutherford.
(ii) The nucleus is surrounded by electrons that move around the nucleus with a very high speed in circular paths called orbits. Thus, Rutherford’s model of atom resembles the solar system in which the nucleus plays the role of sun and the electrons that of revolving planets.
(iii) Electrons and the nucleus are held together by electrostatic forces of attraction.
The Rutherford’s model explains the structure of atom in a very simple way, but, it suffers from the following drawbacks:
1) Bohr pointed out that Rutherford’s atom should be highly unstable. An electron revolving around the nucleus gets accelerated towards the nucleus. According to the Maxwell, electromagnetic theory, an accelerating charged particle must emit radiation, and lose energy. Because of this loss of energy, the electron would slow down, and will not be able to withstand the attraction of the nucleus. As a result, the electron should follow a spiral path, and ultimately fall into nucleus. If it happens then the atom should collapse in about 10-8 second. But, this does not happen: atoms are stable. This indicates that there is something wrong in the Rutherford’ mass nuclear model of atom.
2) The Rutherford’s model of atom does not say anything about the arrangement of electrons in an atom.
To solve this problem Neils Bohr proposed an improved form of Rutherford’s atomic model called the Rutherford–Bohr model or just Bohr model for short (1913).
4) Isotopes: Atoms of an element with the same atomic number but different mass number.
5) Isobars: Atoms, having the same mass numbers but different atomic numbers, e.g.,
6) Isotones: Atoms having the same number of neutrons but different number of protons or mass number for example
Rutherford presented his own physical model for subatomic structure, as an interpretation for the unexpected experimental results. In it, the atom is made up of a central charge (this is the modern atomic nucleus, though Rutherford did not use the term "nucleus" in his paper) surrounded by a cloud of (presumably) orbiting electrons. In this May 1911 paper, Rutherford only commits himself to a small central region of very high positive or negative charge in the atom.
in this experiment a stream of high energy α–particles from a radioactive source was directed at a thin foil (thickness ∼ 100 nm) of gold metal. The thin gold foil had a circular fluorescent zinc sulphide screen around it. Whenever α–particles struck the screen, a tiny flash of light was produced at that point.
He was observed that :
(i) most of the α– particles passed through the gold foil without deflected.
(ii) a few of the α–particles was deflected by small angles.
(iii) a very few of the α– particles (∼1 in 20,000) bounced back, that is, were deflected by nearly 180°.
On the basis of the observations, Rutherford drew the following conclusions regarding the structure of atom :
(i) Most of the space in the atom is empty as most of the α–particles passed through the foil without deflected.
(ii) A few positively charged α– particles were deflected. The deflection must be due to enormous repulsive force showing that the positive charge of the atom is not spread throughout the atom as Thomson had presumed. The positive charge has to be concentrated in a very small volume that repelled and deflected the positively charged α– particles.
(iii) Calculations by Rutherford showed that the volume occupied by the nucleus is negligibly small as compared to the total volume of the atom. The radius of the atom is about 10-10 m while radius or nucleus is about 10-15 m.
On the basis of above observations and conclusions, Rutherford proposed the nuclear model of atom (after the discovery of protons). According to this model :
(i) The positive charge and most of the mass of the atom was densely concentrated in extremely small region. This very small portion of the atom was called nucleus by Rutherford.
(ii) The nucleus is surrounded by electrons that move around the nucleus with a very high speed in circular paths called orbits. Thus, Rutherford’s model of atom resembles the solar system in which the nucleus plays the role of sun and the electrons that of revolving planets.
(iii) Electrons and the nucleus are held together by electrostatic forces of attraction.
Drawbacks of Rutherford Model
The Rutherford’s model explains the structure of atom in a very simple way, but, it suffers from the following drawbacks:1) Bohr pointed out that Rutherford’s atom should be highly unstable. An electron revolving around the nucleus gets accelerated towards the nucleus. According to the Maxwell, electromagnetic theory, an accelerating charged particle must emit radiation, and lose energy. Because of this loss of energy, the electron would slow down, and will not be able to withstand the attraction of the nucleus. As a result, the electron should follow a spiral path, and ultimately fall into nucleus. If it happens then the atom should collapse in about 10-8 second. But, this does not happen: atoms are stable. This indicates that there is something wrong in the Rutherford’ mass nuclear model of atom.
2) The Rutherford’s model of atom does not say anything about the arrangement of electrons in an atom.
To solve this problem Neils Bohr proposed an improved form of Rutherford’s atomic model called the Rutherford–Bohr model or just Bohr model for short (1913).
Lets take a look on some atomic terms:
1) Nucleons: Sub-atomic particles in the nucleus of an atom, i.e., protons and neutrons.
2) Atomic number (Z): The number of protons in the nucleus of an atom and this is equal to the number of electrons in the atoms because of stability.
Atomic Number (Z) = number of protons in the nucleus of an atom = number of electrons in a nuetral atom.
3) Mass number (A): Sum of the number of protons and neutrons, i.e., the total number of nucleons.
mass number (A) = number of protons (Z) + number of neutrons (n)
4) Isotopes: Atoms of an element with the same atomic number but different mass number.
for example hydrogen has three isotopes that is protium (P), deuterium (D) and tritium(T ).
5) Isobars: Atoms, having the same mass numbers but different atomic numbers, e.g.,
6) Isotones: Atoms having the same number of neutrons but different number of protons or mass number for example
7) Isoelectronic species: Atoms molecules or ions having the same number of electrons, e.g., N2, CO, CN- etc.



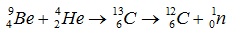






No comments:
Post a Comment