LEC 9 Shapes of Atomic Orbitals
The orbital wave
function or ψ for an electron in an atom has no physical meaning. It is simply
a mathematical function of the coordinates of the electron but the square of the wave function (i.e.,ψ2) at a point gives the probability density of the electron at
that point.
An orbitals is the region of space around the nucleus within which the probability of finding an electron of given energy is maximum above 90%. the shape of this region (electron cloud) gives the shape of the orbital that determine by the azimuthal quantum number (l) while the orientation of the orbitals depends on the magnetic quantum number (m). lets now see the shape of orbitals in the various subshells.
An orbitals is the region of space around the nucleus within which the probability of finding an electron of given energy is maximum above 90%. the shape of this region (electron cloud) gives the shape of the orbital that determine by the azimuthal quantum number (l) while the orientation of the orbitals depends on the magnetic quantum number (m). lets now see the shape of orbitals in the various subshells.
1) S-Orbitals (l=0)
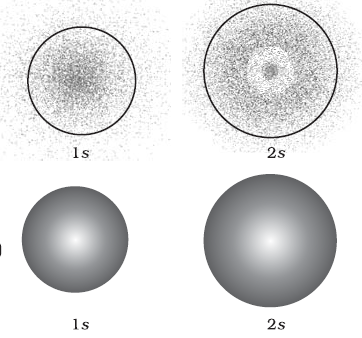
The variation of ψ2 as
a function of r for 1s and 2s orbitals is given as
It may
be noted that
1) for 1s orbital the probability density is maximum at the nucleus
and it decreases sharply as we move away from it. On the other hand, for 2s
orbital the probability density first decreases sharply to zero and again
starts increasing. After reaching a small maxima it decreases again and
approaches zero as the value of r increases further. The region where this
probability density function reduces to zero is called nodal surfaces or simply
nodes. In general,
2) it has been found that ns-orbital has (n – 1) nodes, that
is, number of nodes increases with increase of principal quantum number n. In
other words, number of nodes for 2s orbital is one, two for 3s and so on but there is no nodes for 1s orbitals.
3) boundry surface diagram of constant probability density for
different orbitals give a fairly good representation of the shapes of the
orbitals. In this representation, a boundary surface or contour surface is
drawn in space for an orbital on which the value of probability density |ψ|2 is constant or more than 90%.
4) we see that 1s and 2s orbitals are spherically symmetric, that is, the probability of finding
the electron at a given distance is equal in all the directions. It is also
observed that the size of the s orbital increases with increase in n, that is,
4s > 3s > 2s > 1s and the electron is located further away from the
nucleus as the principal quantum number increases.
2) P-orbitals (l=1)
1) unlike s-orbitals, the boundary surface diagrams are not spherical.
Instead each p orbital consists of two sections called lobes that are on either
side of the plane that passes through the nucleus. The probability density
function is zero on the plane where the two lobes touch each other.
2) for l=1 the value of m is three that is -1, 0, 1 so P orbitals have three different orientation and they are given the designations 2px, 2py,
and 2pz as lobes may be considered to lie along the x, y or z axis.
3) p orbitals increase in size and energy with increase in the principal
quantum number and hence the order of the energy and size of various p orbitals
is 4p > 3p > 2p.
4) the probability density functions for
p-orbital also pass through value zero, besides at zero and infinite distance,
as the distance from the nucleus increases. The number of radial nodes are given by
the n-–2, that is number of radial node is 1 for 3p orbital, two for 4p orbital
and so on, while l gives angular node that is 1 in case all of p orbitals means total nodes in 3p orbitals are 2 and in 4 p orbitals 3.
3) d-orbitals (l=2)
1) They have relatively complex geometry for l=2 we have five value of m that is -2, -1, 0, 1, 2 means d orbitals have 5 different orientation and they are given designation as
2) The shapes of the first four d-orbitals are similar to each other, where as that of the fifth one, dz2 is different from others. all five 3d orbitals are equivalent in energy. The d
orbitals for which n is greater than 3 (4d, 5d…) also have shapes similar to 3d
orbital, but differ in energy and size.
Radial and Angular
Nodes
There are two types of
nodes that can occur; angular and radial nodes. An angular node is a flat plane, The ℓ quantum number determines
the number of angular nodes an orbital will have. A radial node is a circular
ring that occurs as the principle quantum number increases. Thus, n tells us
how many radial nodes an orbital will have and is calculable with the equation:
Total Number of Nodes = n-1.
Number of Angular Nodes = l.
Number of radial Nodes = Total Nodes - Angular Nodes
For
example, let us determine the nodes in the 3pz orbital. We are given that n = 3
and ℓ = 1 because of the p orbital. We can determine the total number of nodes
present in this orbital because: nodes = n-1. In this case, 3-1=2, so there is
a total of 2 nodes. The quantum number ℓ tells us how many angular nodes there
are, so there is 1 angular node, specifically on the xy plane because this is a
pz orbital. Since there is one node left, there must be one radial node. To sum
up, the 3pz orbital has 2 nodes: 1 angular node and 1 radial node.
For example, energy of 2s orbital of hydrogen atom is greater than that of 2s orbital of lithium.
Energies of Orbitals
For example, energy of 2s orbital of hydrogen atom is greater than that of 2s orbital of lithium.
1) The
energy of an electron in a hydrogen atom is determined solely by the principal
quantum number. Thus the energy of the orbitals increases as follows :
1s < 2s = 2p < 3s = 3p = 3d < 4s = 4p = 4d = 4f <
The shapes of 2s and 2p orbitals are different,
an electron has the same energy when it is in the 2s orbital as when it is
present in 2p orbital. The orbitals having the same energy are called degenerate.
2) The energy of an electron in a multielectron atom, unlike
that of the hydrogen atom, depends not only on its principal quantum number
(shell), but also on its azimuthal quantum number (subshell). That is, for a
given principal quantum number, s, p, d, f … all have different energies.
The
main reason for having different energies of the subshells is the mutual
repulsion among the electrons in a multi-electron atoms. The
only electrical interaction present in hydrogen atom is the attraction between
the negatively charged electron and the positively charged nucleus. In
multi-electron atoms, besides the
presence of attraction between the electron and nucleus, there are repulsion
terms between every electron and other electrons present in the atom. Thus
the stability of an electron in multi-electron atom is because total attractive
interactions are more than the repulsive interactions.
3) On the other hand, the attractive interactions of an electron
increases with increase of positive charge (Ze) on the nucleus. the energy of interaction between, the
nucleus and electron (that is orbital energy) decreases (that is more negative)
with the increase of atomic number (Z). Hence that energies of the orbitals
in the same subshell decrease with increase in the atomic number (Zeff ).
4) Both the attractive
and repulsive interactions depend upon the shell and shape of the orbital in
which the electron is present. Further due to spherical shape, s orbital
electron spends more time close to the nucleus in comparison to p orbital and p
orbital spends more time in the vicinity of nucleus in comparison to d orbital.
In other words, for a given shell (principal quantum number), the Zeff experienced by the orbital decreases
with increase of azimuthal.
5) The repulsive interaction of the electrons in the outer shell
with the electrons in the inner shell are more important. . Due to the presence of electrons in the
inner shells, the electron in the outer shell will not experience the full positive
charge on the nucleus (Ze), but will be lowered due to the partial screening of
positive charge on the nucleus by the inner shell electrons. This is known as
the shielding of the outshell electrons from the nucleus by the inner shell
electrons, and the net positive charge experienced by the electron from the
nucleus is known as effective nuclear
charge (Zeff e).
The order of shielding effect is s>p>d>f means being
spherical in shape, the s orbital shields the electrons from the nucleus more
effectively as compared to p and d orbital, even though all these orbitals are
present in the same shell.
6) Since the extent of shielding of the nucleus is different for
different orbitals, it leads to the splitting of the energies of the orbitals
within the same shell (or same principal quantum number), that is, energy of
the orbital, as mentioned earlier, depends upon the values of n and l.
Mathematically, the dependence of energies of the orbitals on n and l are quite
complicated but one simple rule is that of combined value of n and l. The lower the value of (n + l) for an
orbital, the lower is its energy. If two orbitals have the same value of (n +
l), the orbital with lower value of n will have the lower energy.
It
may be noted that different subshells of a particular shell have different
energies in case of multi-electrons atoms. However, in hydrogen atom, these
have the same energy.
Filling of Orbitals in Atom
The
filling of electrons into the orbitals of different atoms takes place according
to the aufbau principle which is based on the Pauli’s exclusion principle, the
Hund’s rule of maximum multiplicity and the relative energies of the orbitals.
Aufbau Principle
The principle
states : In the ground state of the atoms, the orbitals are filled in order of
their increasing energies. In other words, electrons first occupy the lowest
energy orbital available to them and enter into higher energy orbitals only
after the lower energy orbitals are filled.
The order in which the energies of the orbitals increase and
hence the order in which the orbitals are filled is as follows :
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 4f, 5d, 6p, 7s…
The order may be remembered by using the method given in the fig. Starting from the top, the direction of the arrows gives the order of
filling of orbitals, that is starting from right top to bottom left
Pauli Exclusion Principle
The number of electrons to
be filled in various orbitals is restricted by the pauli exclusion principle, According to this principle :
No two electrons in an atom can have the same set of four quantum numbers.
Pauli exclusion principle can also be stated as : “Only two electrons may exist
in the same orbital and these electrons must have opposite spin.” This means
that the two electrons can have the same value of three quantum numbers n, l
and ml, but must have the opposite spin quantum number. The
restriction imposed by Pauli’s exclusion principle on the number of electrons
in an orbital helps in calculating the capacity of electrons to be present in
any subshell. For example, subshell 1s comprises of one orbital and thus the
maximum number of electrons present in 1s subshell can be two, in p and d
subshells, the maximum number of electrons can be 6 and 10 and so on. This can
be summed up as : the maximum number of electrons in the shell with principal
quantum number n is equal to 2n2.
Hund’s Rule of Maximum Multiplicity
This rule deals with the filling of electrons into the orbitals
belonging to the same subshell (that is, orbitals of equal energy, called
degenerate orbitals). It states : pairing of electrons in the orbitals
belonging to the same subshell (p, d or f) does not take place until each
orbital belonging to that subshell has got one electron each i.e., it is singly
occupied.
Since there are three p, five d and seven f orbitals, therefore,
the pairing of electrons will start in the p, d and f orbitals with the entry
of 4th, 6th and 8th electron, respectively. It has been observed that half
filled and fully filled degenerate set of orbitals acquire extra stability due
to their symmetry.
Stability of Completely Filled and Half Filled Subshells
The ground state electronic
configuration of the atom of an element always corresponds to the state of the
lowest total electronic energy. The electronic configurations of most of the
atoms follow the basic rules, However, in certain
elements such as Cu, or Cr, where the two subshells (4s and 3d) differ slightly
in their energies, an electron shifts from a subshell of lower energy (4s) to a
subshell of higher energy (3d), provided such a shift results in all orbitals
of the subshell of higher energy getting either completely filled or half
filled. The valence electronic configurations of Cr and Cu, therefore, are 3d54s1 and 3d10 4s1 respectively and not 3d4 4s2 and
3d9 4s2. It
has been found that there is extra stability associated with these electronic
configurations.
Stability of Completely Filled and Half Filled Subshells
The ground state
electronic configuration of the atom of an element always corresponds to the
state of the lowest total electronic energy. It has been found that there is extra stability
associated with completely filled and half filled electronic configurations because of following reasons:
1) Symmetrical
distribution of electrons:
The
completely filled or half filled subshells have symmetrical distribution of
electrons in them and are therefore more stable. their shielding of one another
is relatively small and the electrons are more strongly attracted by the
nucleus.
2.
Exchange Energy:
The
stabilizing effect arises whenever two or more electrons with the same spin are
present in the degenerate orbitals of a subshell. These electrons tend to
exchange their positions and the energy released due to this exchange is called
exchange energy. The number of exchanges that can take place is maximum when
the subshell is either half filled or completely filled As a result the
exchange energy is maximum and so is the stability.
We can
say that the extra stability of
half-filled and completely filled subshell is due to:
1) relatively small shielding,
2) smaller coulombic repulsion energy, and
3) larger exchange energy.
Details
about the exchange energy will be dealt with in higher classes.
Electronic Configuration of Atoms
The distribution of
electrons into orbitals of an atom is called its electronic configuration. If one keeps in mind
the basic rules which govern the filling of different atomic orbitals, the
electronic configurations of different atoms can be written very easily.
The
electronic configuration of different atoms can be represented in two ways :
(i) sa pb dc ……
notation
(ii)
Orbital diagram
In
the first notation, the subshell is represented by the respective letter symbol
and the number of electrons present in the subshell is depicted, as the super
script, like a, b, c, … etc. The similar subshell represented for different
shells is differentiated by writing the principal quantum number before the
respective subshell.
In the
second notation each orbital of the subshell is represented by a box and the
electron is represented by an arrow (↑) a positive spin or an arrow (↓) a
negative spin. The advantage of second notation over the first is that it
represents all the four quantum numbers.
The electrons in the completely filled shells are known as core
electrons and the electrons that are added to the electronic shell with
the highest principal quantum number are called valence electrons. For example,
the electrons in Ne are the core electrons and the electrons from Na to Ar are
the valence electrons.
We may be puzzled by
the fact that chromium and copper have five and ten electrons in 3d orbitals
rather than four and nine as their position would have indicated with
two-electrons in the 4s orbital. The reason is p3, p6, d5, d10, f7, f14 are fully filled
orbitals and halffilled orbitals have extra stability.
the electronic configuration is show as:
..........END OF THIS UNIT..........









No comments:
Post a Comment